Ingredient bans and restrictions - 2021 update
Upcoming bans, restrictions and ingredients to look out for in 2021.
Natural indigo

What’s happening? Extracts of Indigofera Tinctoria are only permitted at up to 25% in non oxidative hair colour.
When? 3 June 2021 (EU and UK)
Will it affect me? Possibly, if you use natural indigo as a colourant for soaps and bath bombs. You may need to move to synthetic indigo instead.
The SCCS (Scientific Committee on Consumer Safety) reviewed the safety of Indigofera Tinctoria in April 2020 for the purposes of it's use as a hair dye. The review was positive and the committee agreed it could be used for this purpose.
Unfortunately, it was implemented into Annex III, the list of substances that cosmetics are not allowed to use, unless they meet the conditions stipulated. This means that it is ONLY allowed to be used at up to 25% in hair dye products, it is not allowed for other purposes.
You may wonder why this is an issue as only allowed colours are permissible in cosmetic products and Indigofera Tinctoria is not approved for use as a colour. However, a lot of crafters use it in their soaps and bath bombs for its 'skin conditioning properties' and get a blue colour as a secondary effect.
So what are the options? CI 73000, synthetic indigo, is currently allowed as a cosmetic colourant so you could make the switch to that. Please take care to ensure that the grade is pure, as CI 73000 is also used as a clothes dye and can be contaminated with heavy metals and other harmful chemicals.
CI 73000 is also awaiting review by the SCCS, so there is no guarantee that it won't be subject to future bans/restrictions.
Update: A soapmaker recently reached out to the Commission for clarification and they have provided the following response:
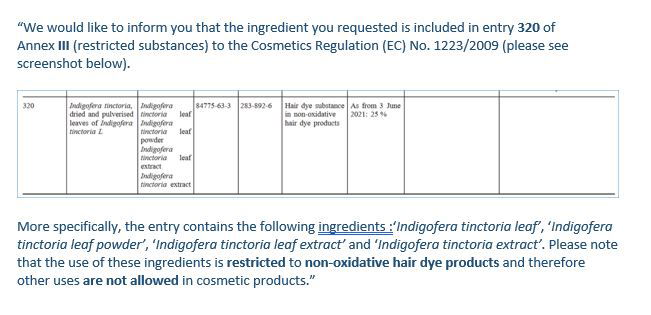
So if you are a soapmaker who uses natural indigo (or uses it for purposed other than non-oxidative dyes), I would recommend removal or substituting with another ingredient.
Butylphenyl Methylpropional

What’s happening? Butylphenyl Methylpropional, a fragrance ingredient, is going to be banned.
When? 1st March 2022.
Will it affect me? Possibly - it is a very popular aroma chemical found in a lot of fragrance oils.
Butylphenyl Methylpropional (also known as BMHCA, Lilial) is a synthetic fragrance that helps to mimic the scent of ‘Lily of the Valley’. Unfortunately, this chemical has also been found to cause reproductive issues in animal studies. In the EU, chemicals which are Carcinogenic, Mutagenic or Reprotoxic cannot be used in cosmetics, unless they meet the exemption criteria.
One of the exclusion criteria is a favourable review of safety from the SCCS (Scientific Committee on Consumer Safety). So far the SCCS has not reviewed this chemical favourably.
In our opinion, it is not likely that this chemical will be reviewed in a positive light in 2021. The worst case scenario is that Butylphenyl Methylpropional will be banned for cosmetics use in the EU cosmetics by 1st March 2022.
Butylphenyl Methylpropional is found in a lot of fragrances, approximately 20% of the fragrances we see contain Butylphenyl Methylpropional. We would strongly recommend you check your existing fragrances and prepare for re-formulation. New products should not be made with fragrances containing Butylphenyl Methylpropional.
With Brexit we are unsure whether the UK government will also implement a ban. However from a practical standpoint, more fragrance houses will seek to remove it meaning that you may be forced to update your CPSR.
Update: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R1902&from=EN
Ban is confirmed for 1st March 2022.

Titanium Dioxide
What’s happening? Titanium Dioxide (Also known as CI 77891) is likely to be classified as a carcinogen (cancer causing ingredient) when inhaled.
When? 9th September 2020
Will it affect me? Possibly. Supply and costs of transport as a result of the classification may have an impact.
Titanium Dioxide is used as a sunscreen, and colourant in cosmetic products. It is also widely used in paints, foods, plastics, paper, and ceramics amongst others. It has been found to cause cancer via inhalation in animal models. This sounds scary, but it is well known that the inhalation of certain powders can cause cancer when inhaled, particularly at high concentrations, and rats are particularly susceptible to this for a number of reasons. Increased cancer risk has not been observed in human studies who have occupational exposure to Titanium Dioxide.
Unlike Butylphenyl Methylpropional, Titanium Dioxide has already been reviewed by the SCCS favourably, as a colourant and for sunscreens, at up to 25%, and for the nano versions of Titanium Dioxide, when used in products which cannot be inhaled.
The SCCS was asked to review Titanium Dioxide in 2020, however, this is where it gets a bit complicated. The SCCS would only comment on products which may be likely to lead to inhalation, on the basis that the Titanium Dioxide was classified as a ‘Carcinogen Category 2 (inhalation)’ for the purposes of CLP Regulation Annex VI entry); this classification applies to TiO2 ’in powder form containing 1% or more of particles with an aerodynamic diameter of ≤ 10 µm’.
It would have been better from a regulatory standpoint if the SCCS had reiterated the safety of products containing Titanium Dioxide which are not likely to be inhaled (i.e. solid and liquid formulations which are not sprayed).
As it stands for cosmetics, some EU Member States may dispute the safety in non-spray products. Where we think the biggest impact will be is actually the effect of the classification on the transport, storage and handling of Titanium Dioxide, this may cause an increase in costs and difficulty in obtaining Titanium Dioxide from suppliers.

HEMA
When? 3rd June 2021
What’s happening? HEMA and DI-HEMA TRIMETHYLHEXYL DICARBAMATE are going to be allowed for professional use only
Will it affect me? It's quite a niche product, but those who cure their own nails may be affected by not being available to buy these products in shops and having to visit a nail salon instead.
HEMA and DI-HEMA TRIMETHYLHEXYL DICARBAMATE are monomers which under UV react to form gel polish and other artificial nail curing systems. Sweden first raised the alarm, with a worrying trend of adverse reactions and Severe Undesirable Effects, related to home cured nail kits. Across the EU, many dermatologists noted that there was a dramatic increase in allergies to HEMA and DI-HEMA TRIMETHYLHEXYL DICARBAMATE.
The problem is that EMA and DI-HEMA TRIMETHYLHEXYL DICARBAMATE are not potent allergens when fully cured, but with sub-optimal curing lamps and non-compliance with instructions, these nails systems were not fully cured, leaving consumers at greater risk of allergy.
The SCCS reviewed these ingredients and concluded that their use should be restricted to professional use only, where adequate training, equipment and advice can be provided.


