Selling your cosmetic product in the EU/UK
What do you need to sell cosmetics in the UK / EU?
This blog will cover some of the main things you will need to sell your cosmetics in the EU/UK.
Firstly – what is a cosmetic?
Sounds like an odd question but it's a common misconception. In the EU cosmetic products are legally defined as;
“any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours.”
This means that the term ‘cosmetic’ can cover everything from make up, soap, shampoos, conditioners, body lotions, toothpaste, body glitter and hair dyes.

In brief - what do you need?
- Appoint a Responsible Person All cosmetics sold in the EU must have a Responsible Person and the responsible person address must appear on the label. Post Brexit it is likely that you will need to appoint an RP in both regions.
- A Cosmetic Product Safety Report (CPSR). All cosmetic products need assessment by a qualified individual. The cosmetics regulations have bans or restrictions on over 1500 chemicals, so you will need to be certain that your safety assessor is up to date with the latest developments. Depending on your safety assessor’s advice you may need to provide the following tests to be able to get a CPSR. The safety assessor will also generally ask for documentation about your raw materials. Don’t worry - we’ll go through all this information so you know exactly what to expect.
- A Product Information File (PIF). This is the minimum amount of information that we need to hold about your cosmetic product. It doesn’t actually have to be a physical file, it can be online, electronic or paper copies, but you need to be able to access it at short notice (within 48 hours).
- Notify your product on the CPNP portal. You will need to notify your product on the CPNP (Cosmetic Product Notification Portal). This is a free online database that you can perform yourself. Post Brexit there will be a UK portal.
- Labelling Your product needs to be labelled according to the EU cosmetic regulations.
- Make your product according to GMP (Good Manufacturing Practice) See PIF section below for more guidance.
Appoint a Responsible Person (RP) Each EU region has a different interpretation for what this entails for small scale cosmetic manufacturers.
The responsible person must be a legal or natural person based in the EU. The responsible person may be a manufacturer, importer, retailer or consultancy service. Post Brexit it is anticipated that an UK Responsible Person will also be required.
The responsible person has the following obligations:
- Ensuring that the cosmetic product is safe.
- Ensuring that the cosmetic product complies fully with the cosmetic regulations.
- Should a product be found to not comply with the regulations, the RP must take corrective actions.
- Should the product be considered to be unsafe, the RP must take corrective actions.
- The RP must cooperate with the authorities.
- The RP must ensure that the CPSR is adequate and kept up to date.
- The RP must ensure that the cosmetic product is notified on the CPNP portal.
- The RP address must appear on the cosmetic label.
- The RP must inform the authorities of any Serious Undesirable Effects.
A Cosmetic Product Safety Report (CPSR)
We’ve covered the CPSR in more detail in another blog, this will cover more of the practical aspects.
https://www.swiftfoxltd.com/blog/the-importance-of-a-cosmetic-product-safety-report-cpsr
Your safety assessor will generally ask for technical documents for the raw materials in your product, such as MSDSs (Safety Data Sheets), COAs (Certificates of Analysis) and specifications. You should be able to get these from your raw material supplier.
For fragrances/essential oils, you may need to submit IFRA (International Fragrance Association) documentation and allergen declarations. You should be able to get these from your raw material supplier.
Your assessor may also require additional testing to assess your product. As a general rule, there are fewer testing requirements for products that don’t contain water as they tend to be more stable and are less likely to promote the growth of microbes.
If you’re not familiar with these tests – don’t worry we will be going into more detail later.
|
|
|
|
| |
| |
| |
As you can see, products that contain water require more testing – this is something you should definitely consider when developing your product. Some pre-made bases may have these tests performed already, it is worth contacting your supplier to find out if this is the case for you.
A water-based product could add over £500 in testing costs as a rough ballpark estimate. This is one of the reasons that self preserving/anhydrous products like soaps, balms and oils are good products for new businesses to start with, they are generally ‘self-preserving’ and usually have have less testing requirements.
Tests don’t always pass, you may need to tweak your formulation and retest them – consider the chance of failure in your budget.
Stability test
I pretty much always recommend performing a stability test for most products. Although some products can be argued to be relatively stable (bath bombs, soaps, and some anhydrous products) I still recommend performing stability for a lot of product types.
But why?
I personally have seen hundreds of examples of instability in products, even in anhydrous products; with signs of instability of clumping, separation, crystallisation, discolouration, etc. These changes are not tolerated well by retailers and consumers alike. Having an unstable product can be very costly with loss of sales, returns and refunds.
Also, worth mentioning that as the temperature gets warmer oil-based formulations may melt, and in winter formulations may harden. Your product should be able to remain roughly the same throughout the seasons – consumers and retailers will expect this!
Performing accelerated stability testing can give you the assurance that your product is stable under most conditions, is stable in most seasons and is durable.
What is accelerated stability? Your product is run through different conditions, light, high temperature, low temperature and any changes are reported. The higher temperatures are used to predict how long your product will be stable for, a 3 month accelerated stability test can predict the lifespan of a product at around 2-3 years.
Preservative Efficacy Test / Challenge Test
Although a lot of preservatives have got a bad reputation in the media, actually the preservatives in cosmetics are very well studied and controlled in the EU. This test measures how well your product can ‘fight off’ bacteria and mould. Without preservatives your product will likely harbour and grow bacteria and mould. This can be really harmful for consumers.
So what is a preservative Efficacy Test? A good way of thinking about it is it mimics someone with very dirty fingers handling your product and measures how quickly your product can subdue or kill microbes. This is particularly important for products which contain water.
A high level of selected species of bacteria and mould will be added to the product and the product will be retested to see whether the level of bacteria and mould declines.
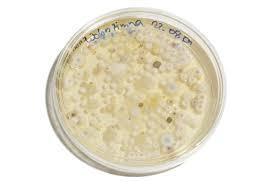
Microbiological testing
You also need to make sure that your product does not contain microbes at harmful levels. Even if a product has passed a challenge test there are occasions where the preservative system can be overwhelmed, allowing harmful microbes to flourish.
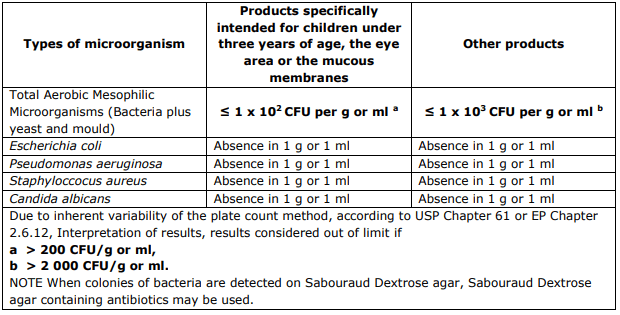
According to the EU SCCS guidelines (SCCS/1564/15) these are the recommended limits:
Product Information File
The word ‘file’ is a little misleading. You don’t actually need a single paper (or computer) file. You do however need to have all this information, on every product, easily to hand, and obtainable within 48 hours. A PIF needs to be kept for up to 10 years.
- Description of the cosmetic product
- CPSR
- Description of manufacturing and statement of compliance
- Proof of claims / claim substantiation
- Data on animal testing
It should be made available in the language of the Member state, so if the Responsible person is in the UK, this would be English.
Below is a link to Cosmetic Europe's guidance if you wish to examine the PIF in more detail:
Description of the cosmetic product
This can be a description of the product, the product’s formulation code/name.
Personally, I also recommend keeping the following information:
CPNP Number(s)
Formulation code/name
Name(s) that the product is sold under
A key thing is to make sure that you can cross reference all the data from your testing/formulations easily.
CPSR
You need to keep an up to date CPSR for your product, although it isn’t explicitly stated in the regulation, I also recommend storing any supporting information required for the CPSR.
Such as;
Technical documentation for your raw materials, such as COAs, MSDSs, IFRA documents etc.
Test results; challenge tests, microbiological testing, and stability tests.
Method of Manufacture and Statement of GMP compliance
All cosmetic products have to be made to GMP. Usually ISO 22716:2007. The ISO guidelines are quite in depth and covers everything from training, maintenance of machinery, cleaning to customer complaints.
Unfortunately, each country in the EU appears to have different interpretations as to what GMP means for small scale manufacturers of cosmetics, with some countries having a more pragmatic approach than others.
This means that you will need to check the requirements for your country.
Not all countries have easily accessible guides, however here are some example guides:
Germany: Checklist for Self Assessment https://www.ikw.org/fileadmin/ikw/downloads/Schoenheitspflege/cosmetic_gmp.pdf
Proof of claims / claim substantiation
Any claims that you make on your product, even relatively simple claims such as ‘moisturising’ will need to have some claim substantiation to support it. This can be information on the ingredients, scientific literature, and any testing that you have performed.
 Unfortunately, 'truth' can be quite subjective!
Unfortunately, 'truth' can be quite subjective!
All claims need to comply with the common criteria:
- Legal compliance
- Truthfulness
- Evidential support
- Honesty
- Fairness
- Informed decision making
For more information see the guidance below:
https://ec.europa.eu/docsroom/documents/24847/attachments/1/translations/en/renditions/native
Some products, such as sunscreen products, will need extensive testing:
http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:265:0039:0043:EN:PDF

Data on animal testing
Any animal testing performed for the purpose of testing the cosmetic products or the ingredients after 11 September 2004. All animal testing was banned for cosmetic purposes after 11th March 2013*.
In order to comply check your supplier’s animal testing declarations, and place a statement that your company and your downline suppliers have not tested the product or the ingredients to the best of your knowledge.
Contrary to popular belief a lot of raw material suppliers are against animal testing. Unfortunately, some regulators are refusing non-animal testing methods. With pressure from animal welfare groups, this situation might change.
*Some readers may be aware of other regulatory requirements, such as REACH may ‘force’ cosmetic ingredient suppliers into testing on animals despite the cosmetic regulations. This is quite a contentious subject, and there are a lot of animal welfare groups that are fighting against these requirements.
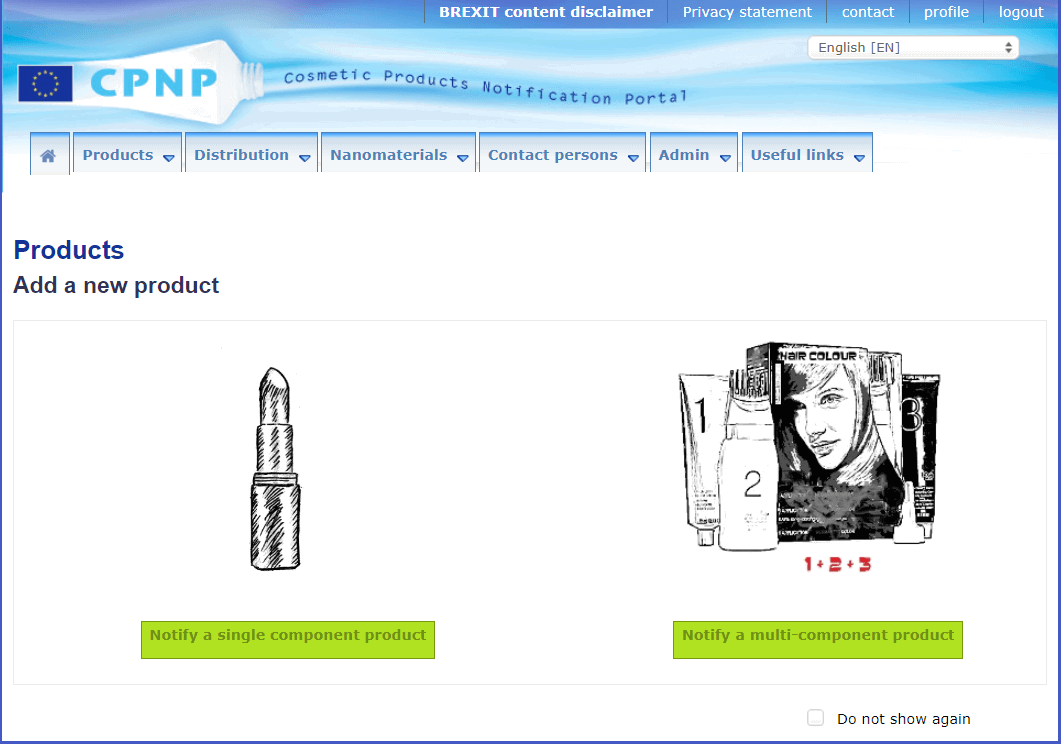
Notify your product on the CPNP portal
All cosmetic products need to be uploaded onto the CPNP portal.
Use the following link to gain access to the CPNP. You will need to log into 3 different systems in order, everytime you log in.
Save the link below as it will help you log into the systems in order:
https://webgate.ec.europa.eu/cpnp/public/tutorial.cfm
Here is the guidance on how to set yourself up:
https://webgate.ec.europa.eu/cpnp/public/saas-start.cfm#saas-step-1
https://webgate.ec.europa.eu/cpnp/public/saas-a2.cfm
You will need your formulation information, a picture/file of your labelling and a picture of the final product in order to upload your product onto the portal.
Thanks for your patience if you have managed to get this far, for any help and guidance with the cosmetic regulations, please get in contact.


