The importance of a Cosmetic Product Safety Report (CPSR)
What is a CPSR?
Prior to placing a cosmetic product into the marketplace in the EU, a Cosmetic Product Safety Report (CPSR) needs to be performed.
Summary:
The CPSR is a essential part of placing your product for sale in the EU. It is paramount that your safety assessor covers the items outlined in this blog. Here we point out the importance of having a safety assessor who delves into the documentation from your raw materials and looks at the impact of trace ingredients/impurities into the safety of your cosmetic product. Information from fragrance allergens from essential oils and fragrance oils should be examined carefully for allergy risk and other safety risks. Your safety assessor should be able to advise you of ways to tweak your product to ensure that it is EU compliant or safer for consumers.
Your safety report should consist of:
Part A
- Quantitative and qualitative composition of the cosmetic product
- Physical/chemical characteristics and stability of the cosmetic product
- Microbiological quality
- Impurities, traces, information about the packaging material
- Normal and reasonably foreseeable use
- Exposure to the cosmetic product
- Exposure to the substances
- Toxicological profile of the substances
- Undesirable effects and serious undesirable effects
- Information on the cosmetic product
Part B
- Assessment conclusion
- Labelled warnings and instructions for use
- Reasoning
- Assessors credentials
Quantitative and qualitative composition of the cosmetic product
This should provide a breakdown of the raw materials in your product, including any impurities or other chemicals which are relevant to the safety assessment.
This sounds simple, however it requires a lot of knowledge of the raw materials and any problematic ingredients.
Here are some examples of ingredients which may have impurities and chemicals which may cause harm.
Carbomer is a polymer which is commonly used to make gels, it also helps with the flow/consistency of products. Carbomer can come in more than one form, one which is made with benzene and one which is not.
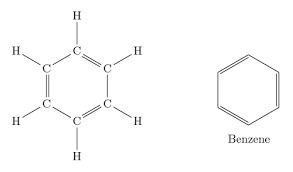
Benzene is banned in cosmetics in the EU (Annex II/47), unless it is 'technically unavoidable', and in those cases it must be kept as low as possible. As you can purchase a non benzene version, it is avoidable. Hopefully you wouldn't want to be placing a prohibited ingredient in your cosmetic product, and your assessor can inform you to make the switch to make your product compliant.

Furocoumarines are naturally occurring chemicals which are found naturally in citrus peel oils. Furocoumarines, when placed on the skin, can enter skin cells, and when exposed to UV light the furanocoumarines can react with the cells DNA. Unfortunately this causes massive damage to the cell, which ultimately can result in cell death.
In more simple terms, furocoumarines are considered to be phototoxic, causing redness, swelling and in some cases, blistering and scarring. These effects can happen at very low concentrations as low as 0.03%.
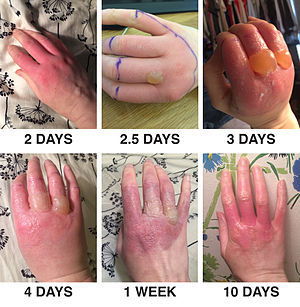
Looking at the picture above (sorry for those of you who are a bit squeamish) this is an example of phototoxicity caused by lime. As you can see phototoxicity can be quite severe, and take some time to resolve.
Thankfully for cosmetic products furocoumarines are prohibited, except for normal content in essential oils and essences. It is restricted to very low levels in sun protection products (Annex II/358). Ideally your safety assessor should check the levels of furocoumarines in your product, especially if your product is exposed to the sun. If the level is too high, they should be able to suggest ways to mitigate the risk (altering raw material source to a furocoumarine free version or alter the concentration in the product).
Fragrance allergens
There are 26 fragrance allergens which must be declared on the label if they are above 0.01% for rinse off, and 0.001% in leave on. Your assessor should look through all of your raw materials for these allergens, and let you know which ones require placing on the label. Here at Swift Fox Consultancy, we like to 'go the extra mile' we also perform QRA allergy assessments, this means that we use the results of animal or more frequently human studies to determine the dose that is unlikely to initiate allergy, we then add additional safety factors and take into account exposure to from other sources. We can then determine a likely 'safe' level, and we make sure that your product falls within that level.
Although it is impossible to completely remove the risk of allergy, especially in people who have preexisting allergies, this gives a greater assurance that your product is less likely to cause allergic reactions.
Physical/chemical characteristics and stability of the cosmetic product
This section takes into account the physical chemical characteristics of the product. This means the physical characteristics of the product, such as whether it is a solid, or liquid. Does it have a characteristic scent, colour?
Stability is also considered. I advise nearly all my clients to perform stability testing on their cosmetic products. Experienced formulators can even perform it themselves. But why is it so important?
From a safety point of view, stability testing can pick up if there are changes/reactions going on in your product, I'll give two examples of when this can impact safety.
Nitrosamines are cancer causing chemicals, which are banned in the EU for good reason. However - did you know that nitrosamines can be formed in cosmetic products. Put simply, if you have the right conditions, trace contaminants/ingredients can react together to form this rather 'nasty' chemical.
Another consideration is allergy risk, some allergens require activation to become more potent allergens, an example of this is limonene. If your product is becoming unstable, this could be a potential risk.
Another reason for performing stability is that, combined with your microbiological test results/risk assessment, it can be used to assess the longevity of your product, and may enable you to use the Period After Opening symbol.

Ever walk into a shop and see an old bottle of discolored product at the back of a shelf? What impression does that give you into the quality of the brand? Is this the impression that you want to give to your customers? In some cases of more naturally derived products, labeling to inform your customer of a small amount of natural variation in color may mitigate some customer complaints by setting expectations.
The stability test also protects you against some quality complaints, if you have demonstrated that your product is stable under a number of conditions you can use these results to the defense of your product. It may indicate the product wasn't stored as recommended. Obviously all quality and safety complaints should be taken seriously and investigated to the fullest extent.
Microbiological quality
This section should cover the microbiological risk of the product. Bacteria and mold can cause severe eye and skin infections and are tightly controlled in the EU.
Some products are considered to be 'self preserving' such as; high alcohol products and soaps, and some products are lower risk, because they lack water (anhydrous).
For higher risk products a Challenge Test or Preservative Efficacy Test (PET) may be performed. Simply put it measures how well your product 'fights off' bacteria and yeast. Or another way of putting it is it tests how well your preservative system is at controlling microbes.
You may also need to test your product per batch, for any present microbes. Even if your product has passed a PET test, contamination of your raw materials or process may 'overwhelm' your preservative system and lead to excessive microbe growth. The SCCS guidance lists the acceptability criteria for microbial growth in cosmetic products.
Your assessor should take all these elements into account when assessing your microbial risk.
Impurities, traces, information about the packaging material
As stated earlier in the blog, trace/impurities in your raw materials can make a big difference to the safety and regulatory of your cosmetic products.
Your safety assessor should also assess the packaging material for compatibility with your cosmetic product and trace ingredients which may be a safety concern. For example rubber components (such as eyelash wands) have been found to leech/react to form nitrosamines.
Normal and reasonably foreseeable use
Your assessor should assess how your customer is likely to use your product. Where will the product be applied? Is it likely to accidentally enter the eyes? Will the product be ingested in small amounts (lip balms)? What are the risks of application? This section should cover the normal and likely use of the product.
Exposure to the cosmetic product
Your assessor should state the target consumer, the amount of product that is likely to be used, how often, the area of the body used and any values that are used in safety calculations.
Exposure to the substances
This should outline the overall exposure to the substances/ingredients in your cosmetic product and any calculations regarding the safety of your product. Your safety assessor should examine the likelihood of your product causing harm as a sum of all the ingredients.
One element which is particularly important in exposure is systemic exposure.
Systemic toxicity: This is the action of a chemical(s) on organ systems. For instance a chemical may have effects on the liver. How does your safety assessor determine whether your product is safe? 
Using a simple example, ingredient X was tested in animals in a historic test performed in 1984. Rats fed ingredient X for 2 years found liver damage at 5000 mg/kg bw/day but at 2000 mg/kg bw/day there were no effects seen. So the 'safe dose' in rats would be considered to be 2000 mg/kg bw/day. But how does this relate to humans? To cover the uncertainty, we use safety factors or uncertainty factors. For example we would use a factor of 100 as an estimate of a 'safe dose' for humans.
2000/100 = 20 mg/kg bw/day or 1.2 g per day for an adult. To put this in perspective a face cream is used at around 1.54 g per day.
The safety assessor will look at whether the ingredient is likely to be ingested while using your product or whether it can go through the skin.
So in this example chemical X is in the product (face cream) at 1% , this is 0.26 mg/kg bw/day. We're assuming all if goes through the skin, even though that is probably unlikely.
So if we take 20 divide it by 0.26 we get 77 as a margin of exposure, this is on top of the 100 fold safety factor already applied. This means we are 177 times under the 'safe dose' seen in animal testing.
We tend to use what we call 'conservative estimates' this means if we have a range of studies, we tend to choose the lowest (most toxic) value to assess safety, we use the highest estimates of product use, we may assume 100% of the ingredient goes through the skin, this gives us a worst case estimate for safety on top of safety factors.
Toxicological profile of the substances
Here your safety assessor should outline the safety information on the ingredients, for example;
- Skin irritation
- Eye irritation
- Allergy
- Systemic toxicity (For example: cancer, organ damage, reproductive damage)
- Phototoxicity (damage caused by exposure to light)
- Genotoxicity (damage to DNA)
Data should comply with the EU animal testing ban. These days there is a lot more in vitro non-animal laboratory tests available.
Undesirable effects and serious undesirable effects
This is a really essential part of the report. If your product is new, you may not have any data on this. However, any reactions seen in clinical trials or patch test data may be used to assess the safety of the product.
If your product has been on the market your safety assessor will expect to be notified of adverse reactions that have been reported to you and the number of units on the market/sold.
Information on the cosmetic product
This section outlines any other aspects which are relevant to the safety of your cosmetic product safety.
Assessment Conclusion
This will outline the aspects from section A and come to a conclusion on the safety of the cosmetic product.
Labelled warnings and instructions for use
This section should cover the legally defined label instructions plus any other recommended label instructions/warnings.
Reasoning
The reasoning behind the assessment conclusion into safety.
Assessors credentials
Your safety assessor needs to have the appropriate qualifications and experience in order to assess cosmetic products. Here at Swift Fox our assessor is a European Registered Toxicologist (ERT) and a Royal Society of Biology Chartered Biologist with extensive experience in the assessment of cosmetic products.
Hopefully you can see just some of the work that goes into assessing your safety of your cosmetic product, and how important it is to ensure that your product is assessed in its entirety, in particular looking at the impurities/trace chemicals of the raw materials and the impact it can have on the safety of your cosmetic products.


